HyStem Thiolated Hyaluronic Acid
We recommend using a Collagenase/Hyaluronidase mixture from Stemcell Technologies found here:
https://www.stemcell.com/products/collagenase-hyaluronidase.html
Catalog #07912
Yes, the Extralink concentration can be adjusted to tune the gelation speed and final gel strength of HyStem hydrogels, as shown below. The concentration represents the concentration of the extralink prior to mixing with Glycosil, not the final extralink concentration in the HyStem hydrogel. To clarify, we used a bulk bottle of Extralink https://advancedbiomatrix.com/extralink-pegda.html and solubilized that material at 0.5 - 4%. We then added that more concentrated Extralink to the other HyStem components in the same ratios as listed in the DFU.
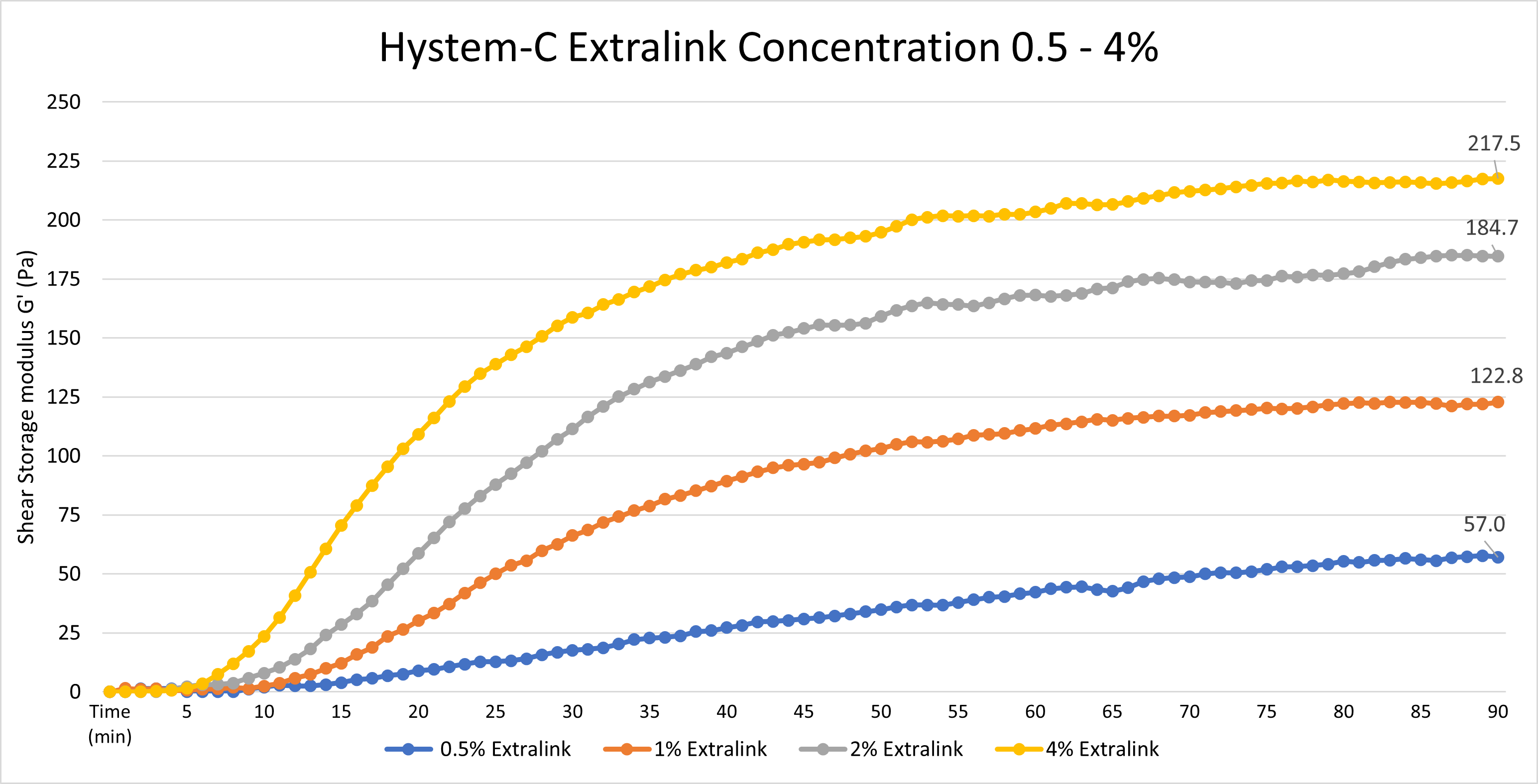
Data was collected using an Elastonsens rheometer from Rheolution: www.rheolution.com
Globular particles less than 75 kDa should be able to freely diffuse through a HyStem hydrogel.
When reconstituted using DG water, the pH of each HyStem component will be approximately 7.4-7.6.
One year from the date of receipt, if stored properly.
Any sterile, deionized, degassed water can be substituted for reconstitution. However, in order to ensure accurate and predictable dissolution and gelation times, our DG Water is highly recommended, as it is degassed, blanketed in argon, and has undergone validation testing with each HyStem component.
Gelin-S provides cellular attachment sites when incorporated in the hydrogel. Gelin-S is thiol-modified, denatured collagen I, derived from either bovine or porcine sources. Gelin-S is included in all HyStem-C and HyStem-HP kits.
Gelin-S has been thiol-modified in the same manner as the hyaluronan in Glycosil (or Heprasil), so that it covalently crosslinks with the Extralink in the HyStem hydrogels.
Yes. Peptides that contain a cysteine residue can be used. The cysteine residue must be present for the peptide to be covalently bonded to the hydrogel substrate.
Yes. ECM proteins, such as laminin, collagen, fibronectin, or vitronectin can be non-covalently incorporated into the hydrogel prior to crosslinking.
HyStem hydrogels and sponges differ in hydration and homogeneity. HyStem sponges are typically polymerized hydrogels that are subsequently freeze-dried. The resulting sponge is a fibrous, mesh network with pores and niches that enable cells to infiltrate and adhere. A true HyStem hydrogel is an encapsulating liquid that polymerizes around suspended cells in culture.
No. The compliance of the hydrogels is set by the amount of Extralink crosslinker added, the concentration of Glycosil (or Heprasil) and Gelin-S used, and the ratio of Glycosil (or Heprasil) to Gelin-S. Once this chemical structure of the hydrogel is fixed, it is not altered by prolonged exposure to cell culture medium.
HyStem sponges can be terminally sterilized by E-beam. HyStem hydrogels have not yet been validated for use with E-beam sterilization methods. HyStem hydrogels are not terminally sterilized by gamma irradiation.
Gelation time is affected by multiple aspects of the gel’s composition.
One way to change the gelation time of a hydrogel is to vary the amount of crosslinker used. Gels with a lower amount of Extralink crosslinker will have a longer gelation time than those with a higher amount of crosslinker. Changing the amount of crosslinker will produce slight changes in gelation time.
Gelation time can be dramatically changed by varying the Glycosil (or Heprasil) and Gelin-S concentrations. Concentrated solutions of Glycosil (or Heprasil) and Gelin-S will create a solution with a much shorter gelation time. This can easily be done by reconstituting the components in a smaller volume of DG Water. Alternatively, diluting these components in larger volumes of DG Water will dramatically increase the total time to form the hydrogel.
HyStem Hydrogels are virtually transparent and should not interfere with microscopy.
HyStem hydrogels may generate mild inflammation as part of the body’s natural healing process in response to injury. HyStem hydrogels do not trigger immune response when used in vivo. (These products are not for human use)
HyStem is degraded in vivo by matrix metalloproteinases (collagenases) and hyaluronidases.
Trypsin, Dipase, collagenase, and hyaluronidase have been used to help detach cells from the surface or from within HyStem hydrogels.
In general, the pore size for HyStem-C and HyStem-HP hydrogels is ~17 nm.
We routinely perform Ellmans test on our Thiolated Hyaluronic Acid. Our product falls between 0.55-0.75 umoles/mg, resulting in a degree of thiolation between 20-30%.
Approximate molecular weight: MW 20000±2000
The approximate molecular weight of the thiolated hyaluronic acid is 300 kDa.
The hyaluronic acid has been thiol-modified on the carboxy groups. These active groups react with acrylate groups on PEGDA, resulting in a hydrogel.
We strongly recommend using the HyStem components the same day that they are solubilized, in order to minimize the chance of autocrosslinking. We cannot guarantee the quality of the product past the first day. If experiments need to be done across two or more days, we recommend leaving the solubilized components at room temperature overnight (capped, in the original vials). We do not recommend re-freezing the components once solubilized.

