-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- RatCol® High Concentration, Solution, 10 mg/ml (rat)
- RatCol® lyophilized, 100 mg (rat)
- RatCol® for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
- PureCol® Collagen Coated Plates
- Collagen Scaffolds
- Collagen Hybridizing Peptides
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft® Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
- Collagen Bioinks for Extrusion Bioprinting
- GelMA Bioinks for Extrusion Bioprinting
- Photoinitiators
- Bioinks and Components for DLP Bioprinting
- Bioink Components
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Methacrylated Polysaccharides
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
HyStem®-IRG UV QuickSet Kit
Photocrosslinkable Thiol-Modified Hyaluronan Kit
Catalog #GS1008
HyStem®-IRG UV QuickSet Kit
Photocrosslinkable Thiol-Modified Hyaluronan Kit
Catalog #GS1008
HyStem®-IRG QuickSet Kits - UV-crosslinkable hyaluronic acid that supports 3D cell culture for tissue engineering and small scale bioprinting applications.
Product Description
Overview
The HyStem-IRG QuickSet Kit is composed of Glycosil® (thiolmodified hyaluronic acid), Gelin-S® (thiol-modified gelatin), PEGCure (PEG-norbornene) and Irgacure 2959 Photoinitiator for photoinitiation. A transparent hydrogel forms after contents are mixed and exposed to UV light (365 nm). All vials are packaged as sterile lyophilized solids that are blanketed by argon and under a slight vacuum.
As one of the first hyaluronic acid-based light-controlled hydrogels, the HyStem®-IRG QuickSet Kit supports 3D cell culture for use in tissue engineering and small scale bioprinting applications. The HyStem®-IRG QuickSet Kit provides increased temporal and spatial control making it ideal for micro-scale bioprinting. Gelation occurs after 30-60 seconds when exposed to ultraviolet light as compared to ~30 minutes using other hydrogel formulations.
Features
- Enables faster, flexible gelation times
- Controlled by UV light
- Optimized for micro-scale bioprinting applications
- Maintains standard characteristics of other HyStem hydrogels
The HyStem® UV QuickSet kit is composed of HyStem (thiol-modified hyaluronic acid), Gelin-S® (thiol-modified gelatin), UVlink™ (PEG-norbornene), Irgacure 2959 for photoinitiation, and DG water. A transparent hydrogel forms when contents are mixed and exposed to UV light. All vials are packaged as sterile lyophilized solids that are blanketed by argon and under a slight vacuum.
Directions for Use
INSTRUCTIONS FOR USE (Download the full directions for use PDF here)
The Irgacure solution is prepared by dissolving the lyophilized solid in DG Water. The Glycosil, Gelin-S, and PEGCure are reconstituted with the Photoinitiator solution. A 7.5 mL hydrogel at 1% (w/v) solution is produced when all reconstituted materials are mixed.
HyStem hydrogels (3 x 2.5 mL = 7.5 mL) should be prepared as follows:
- Allow Glycosil, Gelin-S, PEGCure, irgacure, and DG Water vials to come to room temprerature.
- Under aseptic conditions, use a syringe to add 10.0 mL of DG Water to the Irgacure Photoinitiator vial. Shake or vortex the vial at 37°C for 30 minutes or until fully dissolved.
- Add 1.0 mL of the reconstituted Photoinitiator to the Glycosil vial. Add 1.0 mL of the Photoinitiator solution to the Gelin-S vial.
- Place both vials horizontally on a rocker or shaker. Shake vials at 37°C for 30 minutes or until fully dissolved. It may take up to 60 minutes for the solids to fully dissolve.
Solutions should be clear and slightly viscous.
- Add 0.5 mL of Photoinitiator to the PEGCure vial. Place on a shaker and mix at 37°C for approximately 10 minutes.
- Combine the Glycosil, Gelin-S, and PEGCure solutions and mix well.
- Pipette solution into desired format (i.e. 96 well plate). Using a hand-held UV light source, expose the gel to the UV light (wavelength 365nm) until the gel reaches the desired stiffness. Gelation will occur between 15 seconds and 1 minute. Results may vary depending on the UV source manufacturer and/or plate design
Note: Gelation time and gel stiffness can be adjusted by varying the concentration of Glycosil, GelinS, or UVlink.
Note: Each kit component has been manufactured under aseptic conditions and tested for bacteria and fungus.
Product Q & A
We recommend using a Collagenase/Hyaluronidase mixture from Stemcell Technologies found here:
https://www.stemcell.com/products/collagenase-hyaluronidase.html
Catalog #07912
Yes, the Extralink concentration can be adjusted to tune the gelation speed and final gel strength of HyStem hydrogels, as shown below. The concentration represents the concentration of the extralink prior to mixing with Glycosil, not the final extralink concentration in the HyStem hydrogel. To clarify, we used a bulk bottle of Extralink https://advancedbiomatrix.com/extralink-pegda.html and solubilized that material at 0.5 - 4%. We then added that more concentrated Extralink to the other HyStem components in the same ratios as listed in the DFU.
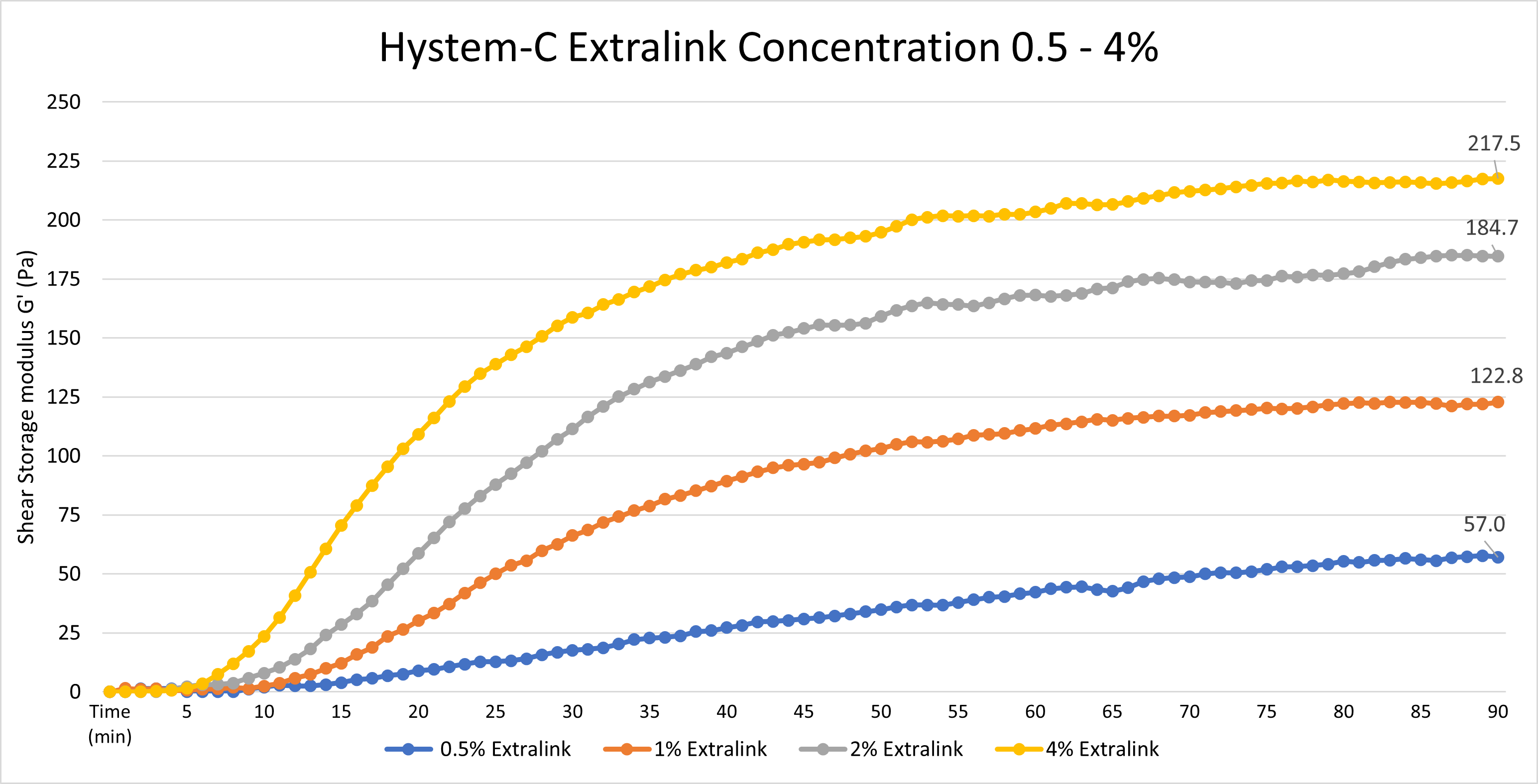
Data was collected using an Elastonsens rheometer from Rheolution: www.rheolution.com
Globular particles less than 75 kDa should be able to freely diffuse through a HyStem hydrogel.
When reconstituted using DG water, the pH of each HyStem component will be approximately 7.4-7.6.
One year from the date of receipt, if stored properly.
Any sterile, deionized, degassed water can be substituted for reconstitution. However, in order to ensure accurate and predictable dissolution and gelation times, our DG Water is highly recommended, as it is degassed, blanketed in argon, and has undergone validation testing with each HyStem component.
Gelin-S provides cellular attachment sites when incorporated in the hydrogel. Gelin-S is thiol-modified, denatured collagen I, derived from either bovine or porcine sources. Gelin-S is included in all HyStem-C and HyStem-HP kits.
Gelin-S has been thiol-modified in the same manner as the hyaluronan in Glycosil (or Heprasil), so that it covalently crosslinks with the Extralink in the HyStem hydrogels.
Yes. Peptides that contain a cysteine residue can be used. The cysteine residue must be present for the peptide to be covalently bonded to the hydrogel substrate.
Yes. ECM proteins, such as laminin, collagen, fibronectin, or vitronectin can be non-covalently incorporated into the hydrogel prior to crosslinking.
HyStem hydrogels and sponges differ in hydration and homogeneity. HyStem sponges are typically polymerized hydrogels that are subsequently freeze-dried. The resulting sponge is a fibrous, mesh network with pores and niches that enable cells to infiltrate and adhere. A true HyStem hydrogel is an encapsulating liquid that polymerizes around suspended cells in culture.
No. The compliance of the hydrogels is set by the amount of Extralink crosslinker added, the concentration of Glycosil (or Heprasil) and Gelin-S used, and the ratio of Glycosil (or Heprasil) to Gelin-S. Once this chemical structure of the hydrogel is fixed, it is not altered by prolonged exposure to cell culture medium.
HyStem sponges can be terminally sterilized by E-beam. HyStem hydrogels have not yet been validated for use with E-beam sterilization methods. HyStem hydrogels are not terminally sterilized by gamma irradiation.
Gelation time is affected by multiple aspects of the gel’s composition.
One way to change the gelation time of a hydrogel is to vary the amount of crosslinker used. Gels with a lower amount of Extralink crosslinker will have a longer gelation time than those with a higher amount of crosslinker. Changing the amount of crosslinker will produce slight changes in gelation time.
Gelation time can be dramatically changed by varying the Glycosil (or Heprasil) and Gelin-S concentrations. Concentrated solutions of Glycosil (or Heprasil) and Gelin-S will create a solution with a much shorter gelation time. This can easily be done by reconstituting the components in a smaller volume of DG Water. Alternatively, diluting these components in larger volumes of DG Water will dramatically increase the total time to form the hydrogel.
HyStem Hydrogels are virtually transparent and should not interfere with microscopy.
HyStem hydrogels may generate mild inflammation as part of the body’s natural healing process in response to injury. HyStem hydrogels do not trigger immune response when used in vivo. (These products are not for human use)
HyStem is degraded in vivo by matrix metalloproteinases (collagenases) and hyaluronidases.
Trypsin, Dipase, collagenase, and hyaluronidase have been used to help detach cells from the surface or from within HyStem hydrogels.
In general, the pore size for HyStem-C and HyStem-HP hydrogels is ~17 nm.
We routinely perform Ellmans test on our Thiolated Hyaluronic Acid. Our product falls between 0.55-0.75 umoles/mg, resulting in a degree of thiolation between 20-30%.
Approximate molecular weight: MW 20000±2000
The approximate molecular weight of the thiolated hyaluronic acid is 300 kDa.
The hyaluronic acid has been thiol-modified on the carboxy groups. These active groups react with acrylate groups on PEGDA, resulting in a hydrogel.
We strongly recommend using the HyStem components the same day that they are solubilized, in order to minimize the chance of autocrosslinking. We cannot guarantee the quality of the product past the first day. If experiments need to be done across two or more days, we recommend leaving the solubilized components at room temperature overnight (capped, in the original vials). We do not recommend re-freezing the components once solubilized.
Product Applications
Read our HyStem eBrochure Here
or
General Notes for Bioprinting with HyStem®-IRG QuickSet Kit:
The following guidelines were developed for a modified Bioforce Nano eNabler™ and exact requirements and parameters will vary between platforms and applications. While other platforms have not been evaluated, any system capable of liquid handling and amenable to UV exposure should be easily adaptable for the HyStem®-IRG QuickSet matrix.
• High relative humidity (>90%) is required in the printing chamber to prevent desiccation of printed constructs.
• If printing live cells, verify that the surface patterning tool or print head is large enough to accommodate cells (approximately 50-100 µm).
• Where possible, UV/ozone treatment of printing tools to enhance hydrophilic nature of the printing channel materials can improve consistency in printing.
• The matrix is a clear liquid with minimally increased viscosity compared to water at room temperature. Upon exposure to 365nm UV light, rapid polymerization occurs resulting in a viscoelastic solid. A 4W handheld UV lamp held approximately 2 cm from the surface of the gel is sufficient to polymerize a gel in as little as 15 seconds.
• Conducting a small pilot study to determine the appropriate exposure time for a given application is strongly recommended as matrix stiffness is directly correlated to exposure time and can impact survivability, proliferation, and differentiation.
Note: The HyStem®-IRG QuickSet Kit has not been thoroughly evaluated for thick, multilayer constructs.
Product Certificate of Analysis
No result for .
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.